본 글은 다음의 두 논문을 참고하여 학부연구생 인턴을 하는 6개월 동안 연구했던 Bio-Adhesive하고 Conductivity를 가지는 Tough Hydrogel에 관한 프로젝트 내용입니다.
<Introduction>
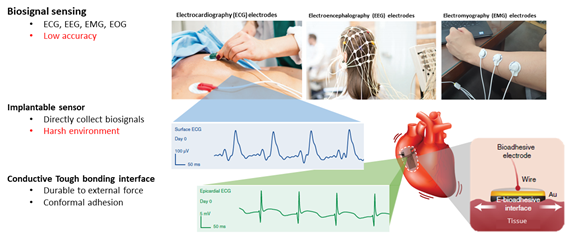
Recently, interest for point of care diagnosis which enable customized diagnosis for each individual patient is increased. Therefore, accurate biosignal sensing by bioelectronics, such as ECG, EEG, EMG, became important issue for precise diagnosis. Even though epidermal sensors has advantages of easy management, it has low accuracy on detecting biosignal compared to implantable sensors which could receive precise data by directly collecting biosignals. However, harsh environments such as dynamic moving and wet tissues disturb data sensing. Therefore, conductive, and conformal adhesive interface between device and tissue is significant to achieve precise sensing. So, I decided to synthesize materials to form conductive and tough bonding bioadhesive interface.
<Background: Tough Bonding>
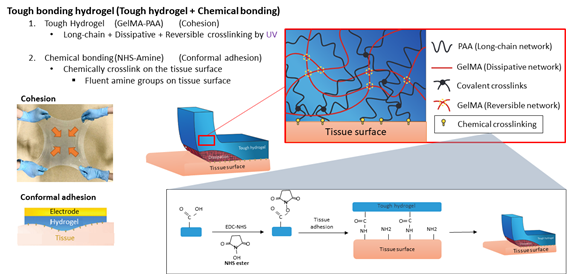
Tough bonding hydrogel is composed with two properties, cohesion, and conformal adhesion. Cohesive network is formed by combining long chain network, dissipative network, and reversible crosslinking network. I synthesized Poly acrylic acid, ‘PAA’ in short, for long chain network, and GelMA for dissipative and reversible network by UV crosslinking method because, GelMA becomes gel by crosslinking methacrylate groups with UV light and acrylic acid polymerizes by heat and UV. Furthermore, conformal adhesion is hard on internal tissues only with physical adhesion because of harsh environments. Therefore, chemically bonding on tissue surface is required for conformal adhesion. Since it is well known that tissue surface contains fluent amine groups, I added N-Hydroxysuccinimide ester, NHS ester in short, which crosslinks between carboxyl groups of PAA and amine groups. The EDC-NHS reaction assists crosslinking NHS ester to carboxyl groups by activating the carboxyl groups.
<Background: CNT for Conductive Network>
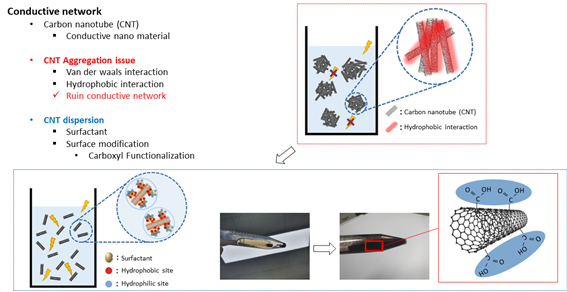
With these theories, I formed tough bonding hydrogel. And now I added carbon nanotube, which is well known for conductive nano material, to form conductive network inside the hydrogel. However, because of ‘van der waals interaction’ and ‘hydrophobic interactions’ such as pi-pi interaction, CNT aggregates when mixed with hydrophilic liquids and could ruin conductive network. Therefore, in order to disperse CNT and form conductive network, researchers added surfactant or modified surface of CNT. Here, I used carboxyl functionalized CNT so that I could easily disperse CNT.
<GelMA: Tough Bonding Experiment>
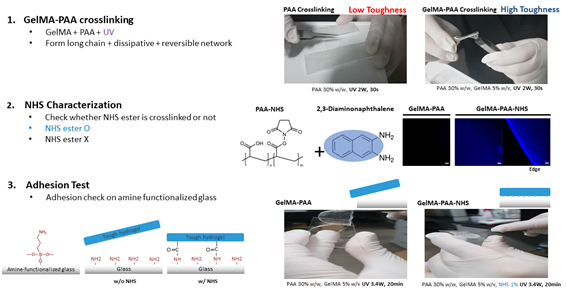
Firstly, GelMA, PAA, and NHS ester were chosen to form tough bonding hydrogel. First of all, I crosslinked GelMA and PAA by UV light. It could not form tough hydrogel without GelMA because dissipative and reversible network is not formed. However, when GelMA and PAA is crosslinked together, cohesive network is constructed. Next, I check whether NHS ester is crosslinked or not by diaminonaphthalene which has amine groups and fluorescent blue light. When NHS ester is not crosslinked, there was no fluorescent on the surface. However, when NHS ester is crosslinked by EDC-NHS reaction, there was fluorescent on the overall surface of the hydrogel. Therefore, I concluded that our EDC-NHS reaction successfully crosslinked NHS ester and therefore I checked adhesion on the amine functionalized glass. Without NHS, the hydrogel easily detached from the glass because there was no chemical bonding. However, hydrogel with NHS ester chemically bonded to amine groups on glass and required much more force to detach it.
<GelMA: Search for Alternative>
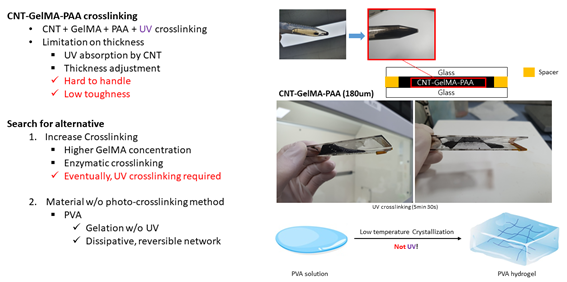
Then, to form conductive network, I dispersed CNT and then mixed CNT solution with GelMA-PAA solution. However, there was limitation on thickness because of the fact that CNT absorbs UV light. Therefore, achieving more than 300µm thickness is difficult and some problems such as handling difficulty and low toughness was observed. Therefore, I searched for alternative ways to increase crosslinking of GelMA. There were various methods such as increasing GelMA concentration, lowering temperature, and adding enzyme to enhance crosslinking. However, these methods all eventually required UV crosslinking method and thus I searched for alternative material which does not require photo-crosslinking method. Finally, I chose poly vinyl alcohol(PVA) which crystalizes at low temperature and form gel. Also, it could substitute role of GelMA in tough bonding hydrogel. Therefore, I synthesized and checked properties of the conductive bioadhesive with PVA.
<PVA: Search for Alternative>

First, I synthesized CNT, PVA, and PAA to form tough hydrogel. I dispersed carboxyl functionalized CNT in DI water and mixed with PVA solution. After mixing CNT and PVA, I crystalized at freezer and formed hydrogel. To add long chain network by PAA, I dried the hydrogel and immersed in acrylic acid solution to infiltrate the acrylic acid monomer into dried and porous polymer. Finally, CNT-PVA-PAA was crosslinked by heat and formed long-chain, dissipative and reversible network for tough hydrogel. Next, I crosslinked NHS ester and checked the adhesion on amine functionalized glass and real skin. Hydrogel without NHS ester easily detached but hydrogel with NHS ester firmly sticked to glass. Finally, I check on real skin (my hand) to check whether it forms conformal contact and stably attached on dynamic movement.
<PVA: Conductivity Experiment>
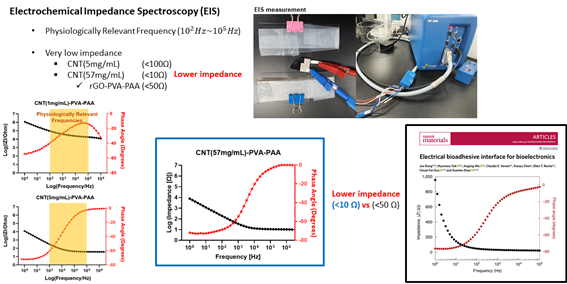
Finally, I measured impedance of the conductive bioadhesive by potentiostat. Results are shown in the graphs. When I increased the concentration of the CNT to 5mg/mL, I could observe low impedance under 100ohm at physiologically relevant frequency. Furthermore, I increased CNT concentration to 57mg/mL to check how low impedance that our conductive bioadhesive could receive. I measured very low impedance under 10ohm with CNT concentration of 57mg/ml which is superior to the graphene oxide base hydrogel from Yuk et al. However, since the CNT has toxicity, high CNT could cause serious damage to tissue so there is trade-off on CNT concentration and biocompatibility.
<Conclusion>
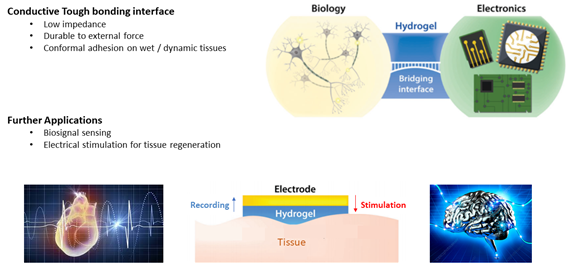
In this research, I developed conductive tough bonding hydrogel interface which has outstanding properties such as low impedance, durable to external force, and form conformal and stable contact on wet and dynamic tissue surface. This interface could be applied on effective biosignal sensing by combining with devices. Furthermore, because of its low impedance, it could be utilized to low power electrical stimulation on electroactive tissue regeneration such as spinal cord stimulation, and neural stimulation.
<국문요약>
본 프로젝트에서는 생체 전자 공학에서 전자 소자와 조직 간의 효율적인 정보 전달 및 접착을 위한 인터페이스를 연구하였습니다. ECG, EEG, EMG와 같은 생체 신호들을 다이나믹한 상황에서도 안정적으로 받아들일 수 있는 터프-하이드로젤을 개발하는 것을 목표로 연구를 시작하였습니다. 먼저, 다양한 물질들을 결합하고 화학적 반응을 응용함으로써 조직표면에 하이드로젤이 보다 안정적으로 달라붙을 수 있는 특성을 부여하였습다. 또한, 각광받고 있는 전도성 나노 물질인 탄소 나노 튜브를 이용해 그래핀과 같은 다른 물질들에 비해 낮은 임피던스를 갖도록 전도성을 부여하였습니다. 그리고 이에 대한 특성들을 측정해보기 위해 다양한 실험들을 진행하였습니다. 먼저, 우리 몸속에 잘 달라붙을 수 있는 지를 확인하기 위해 NHS가 표면에 달라붙은 유리에 하이드로젤을 접촉시켜보았습니다. 그 결과, 일반 유리에서는 잘 떨어지던 하이드로젤이, NHS 표면위에서는 아무리 흔들고, 팅겨보아도 착 달라붙어있는 것을 확인할 수 있었습니다. 다음으로는 전도성 및 임피던스를 측정해보기 위해 Potentiostat을 이용하여 각 주파수 구간에서의 임피던스와 위상을 측정해보았습니다. 그 결과 특히 생체 내의 중요한 정보를 많이 담고 있는 beta dispersion구간에서 매우 낮은 임피던스 값을 확인할 수 있었으며, 기존의 그래핀을 이용하여 측정한 임피던스보다도 더욱 낮은 임피던스값과 함께 높은 전도성을 확인할 수 있었습니다.
<Reference>
1. Yuk, H., et al., Dry double-sided tape for adhesion of wet tissues and devices. Nature, 2019. 575(7781): p. 169-174.
2. Yuk, H., B. Lu, and X. Zhao, Hydrogel bioelectronics. Chemical Society Reviews, 2019. 48(6): p. 1642-1667.
3. Koo, J.H., et al., Wearable electrocardiogram monitor using carbon nanotube electronics and color-tunable organic light-emitting diodes. ACS nano, 2017. 11(10): p. 10032-10041.
4. Ryu, M., et al., Enhancement of interface characteristics of neural probe based on graphene, ZnO nanowires, and conducting polymer PEDOT. ACS applied materials & interfaces, 2017. 9(12): p. 10577-10586.
5. Castagnola, V., et al., Parylene-based flexible neural probes with PEDOT coated surface for brain stimulation and recording. Biosensors and Bioelectronics, 2015. 67: p. 450-457.
6. Han, L., et al., Transparent, adhesive, and conductive hydrogel for soft bioelectronics based on light-transmitting polydopamine-doped polypyrrole nanofibrils. Chemistry of Materials, 2018. 30(16): p. 5561-5572.
7. Rivero, R.E., et al., Pressure and microwave sensors/actuators based on smart hydrogel/conductive polymer nanocomposite. Sensors and Actuators B: Chemical, 2014. 190: p. 270-278.
8. Fidanovski, K. and D. Mawad, Conjugated polymers in bioelectronics: addressing the interface challenge. Advanced healthcare materials, 2019. 8(10): p. 1900053.
9. Deng, J., et al., Electrical bioadhesive interface for bioelectronics. Nature Materials, 2021. 20(2): p. 229-236.
10. Parhi, R., Cross-linked hydrogel for pharmaceutical applications: a review. Advanced pharmaceutical bulletin, 2017. 7(4): p. 515-530.
11. Zhao, X., Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft matter, 2014. 10(5): p. 672-687.
12. Shin, S.R., et al., Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS nano, 2012. 6(1): p. 362-372.
13. Shin, S.R., et al., A bioactive carbon nanotube‐based ink for printing 2D and 3D flexible electronics. Advanced Materials, 2016. 28(17): p. 3280-3289.
14. Shin, S.R., et al., Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS nano, 2013. 7(3): p. 2369-2380.
15. Nabeta, M. and M. Sano, Nanotube foam prepared by gelatin gel as a template. Langmuir, 2005. 21(5): p. 1706-1708.
16. Koh, B. and W. Cheng, Mechanisms of carbon nanotube aggregation and the reversion of carbon nanotube aggregates in aqueous medium. Langmuir, 2014. 30(36): p. 10899-10909.
17. Grimnes, S. and Ø.G. Martinsen. Alpha-dispersion in human tissue. in Journal of Physics: Conference Series. 2010. IOP Publishing.
18. Anderson, D.L. and A.E. Gill, Beta dispersion of inertial waves. Journal of Geophysical Research: Oceans, 1979. 84(C4): p. 1836-1842.
19. Park, H.E., et al., Effect of temperature on gelation and cross-linking of gelatin methacryloyl for biomedical applications. Physics of Fluids, 2020. 32(3): p. 033102.
20. Basara, G., X. Yue, and P. Zorlutuna, Dual crosslinked gelatin methacryloyl hydrogels for photolithography and 3D printing. Gels, 2019. 5(3): p. 34.
'논문 및 소자 리뷰' 카테고리의 다른 글
| E-Drugs (Electronic Drugs): Spatial and Temporal Medical Treatment of Human Disease (0) | 2022.01.11 |
|---|---|
| PPG (Photoplethysmography) (0) | 2021.08.20 |
| Voltage Regulator (전원 레귤레이터) (0) | 2021.07.21 |
| ECG (Electrocardiography) Measuring Circuit [Programming] (0) | 2021.07.10 |
| ECG (Electrocardiography) Measuring Circuit (0) | 2021.07.10 |



